What is embryo banking?
Embryo banking is a valuable tool that helps parents achieve their family plans amidst a variety of factors such as age-related fertility decline, desired genetic screening for targeted familial conditions (PGT-M), desire to preserve fertility after medical treatment such as chemotherapy or for those who wish to preserve the option of expanding their family in the future.
A key question for parents considering embryo banking is, “How many embryo creation cycles should we do, and what family size can we hope for?” This is a complicated question. To help parents navigate the tradeoffs, we created an embryo banking calculator—a tool to help families estimate the size of families that are realistic given their ages, genetic background, and the IVF cycles they’ve planned. You can use it here.

Note: For the purposes of this tool, the mother is assumed to be the source of the eggs used to create embryos, and the father the sperm source. To plan an IVF process which involves donor eggs or sperm, we recommend consulting with a genetic counselor.
Traditional IVF
In a traditional IVF flow, patients produce embryos in a single embryo creation cycle and then transfer those embryos sequentially, if viable ones are created, until they achieve a successful pregnancy.

When attempting to conceive the next child, if a couple does not have remaining frozen embryos from the first cycle, the couple will need to perform additional IVF cycles to produce embryos for additional children.

Subsequent embryo creation cycles often occur two or more years after the first IVF cycle — so a woman who is 35 at the time of her first successful round of IVF may be 37-38 at the time a second IVF cycle is practical, and 40+ by the third. IVF egg retrievals are not possible during pregnancy, and are challenging when a couple has a young child:
- IVF egg retrieval often interferes with breastfeeding — the hormone treatments necessary to stimulate egg production may reduce or eliminate milk supply [1].
- The egg retrieval process is physically taxing for many (for example, an increased risk of ovarian torsion), and difficult in the first year of infancy.
- The clinical process of IVF egg retrieval (5-10 clinic visits, plus the retrieval itself) is often a logistical hardship.
In practice, parents will usually not undergo subsequent embryo creation cycles until a year or more after the birth of a child, or two years between IVF cycles. This timeline may be even further delayed if the family experienced one or more failed transfers or miscarriages before their successful birth.
Age of the mother as a key factor
Unfortunately, the same decline in fertility that affects natural conception applies to IVF — egg quality decreases rapidly after a maternal age of ~35, and is usually very low by age 40.
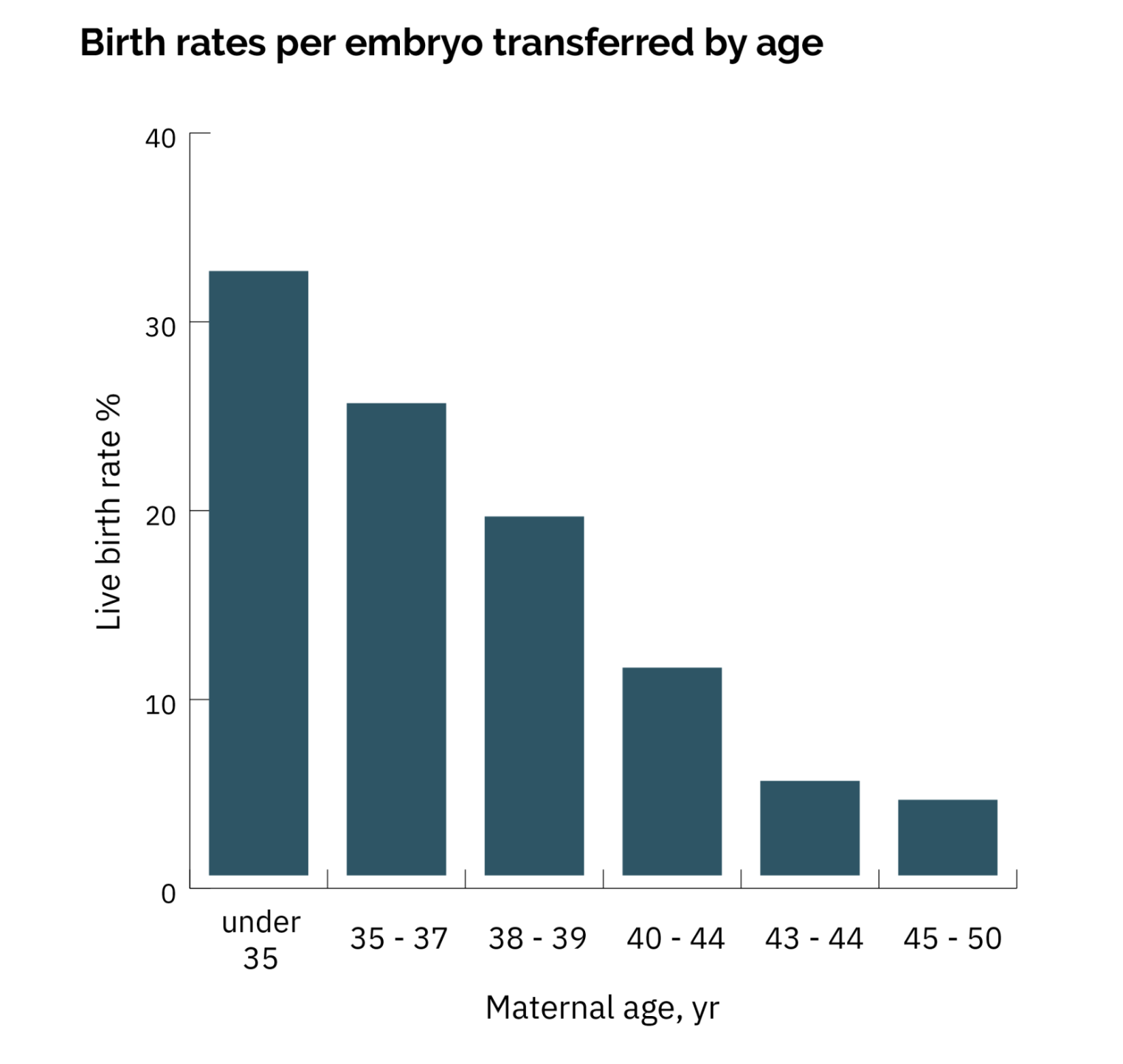
If the first child was conceived when the mother was 35 or older, the mother’s fertility is expected to be significantly lower by the time of the second IVF cycle. As a result, the second IVF cycle will be less likely to end in a successful birth, possibly leaving the parents with a smaller family than they hoped for.
Embryo banking
A couple performs embryo banking when instead of performing an IVF transfer cycle as soon as they produce a viable embryo, they instead perform additional embryo creation cycles to bank embryos until they believe they have enough frozen embryos to reach their target family size.

These retrieval cycles may happen in very quick succession relative to transfer and pregnancy cycles — sometimes even in successive months. The embryos can then be transferred in line with the parents’ preferred birth interval, reducing pressure to conceive the moment it is safe..
However, this brings up an important question for the parents — how many embryo creation cycles should they plan for, and when should they start performing them?
Embryo banking calculator
We've built a calculator to help parents think through this process. The calculator uses the age of the mother at the time of more embryo creation cycles, the availability of existing frozen eggs, and the desire for any targeted monogenic (PGT-M) screening to estimate what family size is realistic given an embryo creation plan.

The simulation estimates for a family:
- The average number of children possible, if the parents sequentially transferred all euploid and unaffected embryos produced during those cycles
- The odds of parents successfully creating a family with at least X children. This is often more relevant than the first number — in a situation where the average family can expect 1 child, through random luck many families would have 2 or more children but many would have none.
- A visualization of the dropoff process, to help parents set realistic expectations at each stage in the IVF process.
Additionally, some families may have frozen eggs stored from earlier fertility preservation or from an egg donor and wonder how those factor in. You can add those, taking into account that ~10% of eggs will be lost during the thawing process.

We use the best estimates of the loss rates at each of the attrition points in the IVF process using recent studies, adjusting for maternal age when it influences the success rate:
- Follicle counts and eggs retrieved per IVF cycle
- Egg maturity rates (the fraction of retrieved eggs usable during IVF)
- Egg fertilization rates using modern ICSI
- The faction of embryos which mature into blastocysts rather than arresting during development
- Aneuploidy rates
- PGT-WGS screening (when using Orchid monogenic and microdup/del screening)
- PGT-M genetic screening
- Transfer success rates
- Pregnancy and live birth success rates
We list the studies and loss rates used in our model at the bottom of page on the calculator itself.
Example family: Embryo banking for fertility preservation
As an example, say a couple where the parents are 32 want to start having children at around 35, but ideally would like three children. They would like to understand how much more effective the embryo banking process would be if they started today, rather than waiting to start until they are ready to conceive at 35. Additionally, the woman has 4 eggs frozen from prior fertility preservation at the age of 31.
We can model the scenario where the couple waited until 35 to perform embryo banking. A single IVF cycle would have poor odds of producing enough transferable embryos for three children:

If the couple underwent an additional round of embryo creation at 35, they would be more likely to bank sufficient embryos to reach their family size:

If the couple underwent embryo creation at 32, on the other hand, they may need only a single embryo creation cycle:

In this situation, the couple may consider starting their fertility and family planning earlier in order to hopefully bank enough embryos to meet their family goals without multiple embryo creation cycles.
Genetic screening and embryo banking
Genetic screening makes the planning more complicated. Preimplantation genetic testing for monogenic disorders (PGT-M) screens for embryos which would be affected by genetic conditions inherited from the parents. Our calculator models the consequences of PGT-M screening in three standard scenarios:
- Screening for recessive conditions (screening 25% of embryos)
- Screening for dominant conditions (50% of embryos)
- Screening embryos for X linked conditions, where 25% or 50% of embryos would be transferred, depending on whether the condition would affect female embryos.
PGT-M screening
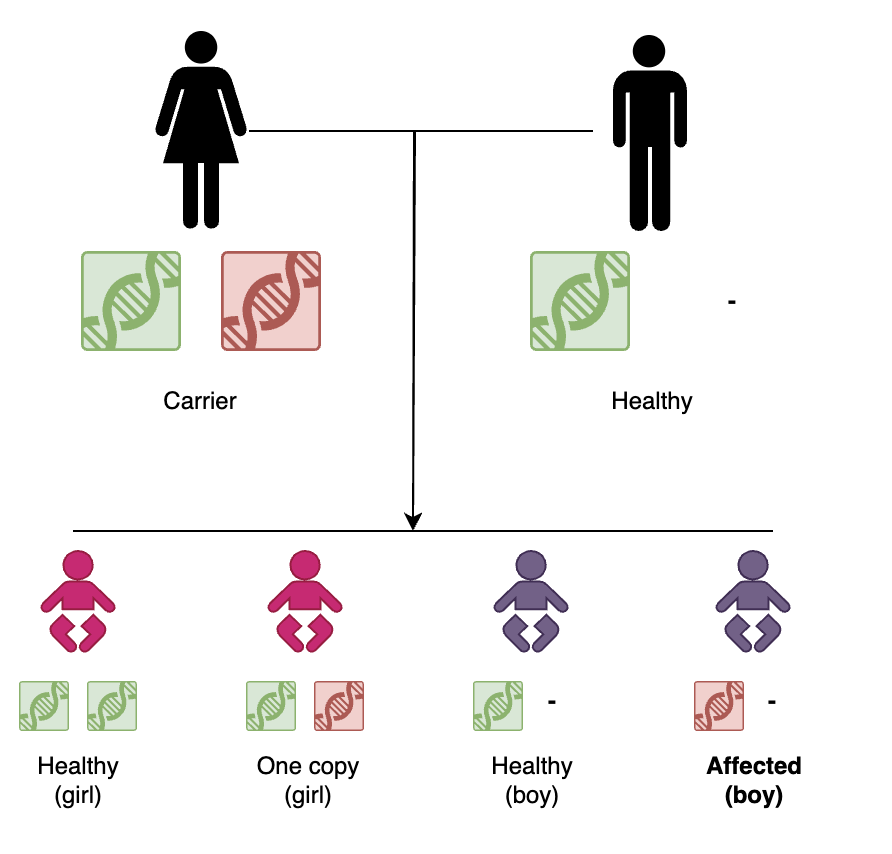
Traditionally, PGT-M screening has primarily been used by parents who share a recessive genetic variant on the same gene. In this situation, the parents are not affected by the disease, but on average 25% (one out of every four) of the embryos would be. This is because the embryo would need to inherit both incorrect copies of a gene in order to inherit the condition:

In recent years, genetic testing has expanded in the breadth of conditions which can be linked to a genetic cause, and one or both parents know that they carry a dominant condition which does not always result in disease (“incomplete penetrance”) — for example, a BRCA mutation which often, but not always, leads to breast cancer. To be affected, a child only needs a single copy of the gene. Many of these parents wish to prioritize the transfer of embryos without these conditions. In this situation, 50% of embryos will inherit the variant and be screened:

The last and most complicated scenario is conditions caused by a gene on the X chromosome (X-linked, or sex-linked, conditions). For example, in the scenario pictured here, the genetic screening will lead to an imbalance in the number of male and female embryos available for transfer after screening, if it’s a condition for which female embryos carrying the variant are usually healthy:
Since males only have a single copy of the X chromosome, in this scenario, only 25% of embryos screen positive during PGT-M, but the remaining embryos will be mostly female, with only 1 male embryo for every 2 female embryos.
Example family: screening for multiple genetic conditions
Our calculator allows parents to model the consequences of screening for one of more of these scenarios, as well as the resulting sex balance.
Let’s say that the parents in our example family from earlier discover during carrier screening that they are share carriers for a hearing loss condition on the GJB2 gene. Additionally, the mother learns she is a carrier for hemophilia A, an X-linked condition on the F8 gene. These parents wish to transfer embryos which are affected by neither condition.

When screening for these conditions, the parents have less than even odds of achieving three children. In this situation, the family may consider two embryo creation cycles, especially if they want a family which includes a boy (in this scenario, they have under a 50% chance of having a male child, with only one third of the screened embryos being male).
Model limitations
This model is based on the best evidence available as of early 2025. However, important limitations exist.
- IVF outcomes are highly individualized. These estimates should be used to guide a conversation with a genetic counselor or physician. These aggregate outcomes are based on a combination of cohort studies. The results for a specific couple will vary based on your IVF center, physician, cycle plan, ovarian reserve and fertility.
- These rates of embryo loss are based on patients who performed IVF for PGT or fertility preservation, not patients who performed IVF due to infertility and/or diminished ovarian reserve. Patients who performed IVF due to infertility are likely to experience higher rates of embryo loss at one or more stages of the IVF flow.
- Limited data exists for the success rates of embryo transfers when the maternal age at transfer is above the early 40s. Success rates of pregnancy will likely be lower for older mothers, regardless of the health of the transferred embryo.
- These outcome statistics are based on a combination of cohort studies. The results for a specific couple will vary based on your IVF center, physician, and cycle plan.
Last and most importantly, just as with natural conception, no amount of planning can guarantee success — regardless of the number of retrievals performed or the parental ages, no fertility plan has a 100% chance of ending in a healthy child.
Ready to start building your family?
This guide helps walk parents through common IVF situations, but every family is different. To talk with a genetic counselor about whether Orchid’s whole-genome embryo screening can help you achieve your family goals, you can schedule a call anytime.
[1] https://carolinasfertilityinstitute.com/can-you-donate-eggs-while-breastfeeding
[2] https://bigfertilityproject.com/what-is-my-chance-of-ivf-success/


-p-500.webp)





