Orchid's team of genetic experts has developed a genetic risk score (GRS) for type 2 diabetes.
Written by Orchid Team
Orchid has developed advanced genetic risk scores (GRS) for a variety of diseases. Here we present our data on our GRS of type 2 diabetes.
Type 2 Diabetes
Type 2 diabetes (T2D) is a chronic metabolic disease in which the body becomes resistant to insulin and gradually fails to maintain normal blood glucose levels. Common symptoms include increased thirst and urination, fatigue, blurred vision, and slow-healing sores, and long-term uncontrolled disease can lead to serious complications involving the heart, kidneys, nerves, and eyes. Major risk factors include obesity, diet, physical inactivity, and age, with disease driven by insulin resistance and impaired insulin secretion.[1][2]
Genetic Risk Score
T2D is shaped by both environmental and genetic factors. Monogenic testing is not available because no single gene causes the condition. Genetic risk scores (GRS), which combine the small effects of many variants into a single score, are currently the only way to estimate genetic risk. Although not diagnostic, a GRS can indicate how likely an individual is to develop the disease.
Orchid’s T2D GRS was trained following current industry standards.[3][4] The GRS was constructed using the SBayesRC algorithm trained on publicly available FinnGen and Million Veterans Program summary statistics.[5][6] The summary statistics include 310,264 cases and 780,665 controls.[7] The resulting GRS contains over a million variants.
Risk predictions are adjusted to each individual’s ancestry, with predictive power decaying as genetic distance from the predominately European training data increases.[8] Orchid considers a GRS meaningfully predictive if individuals at roughly the 97.7th percentile have an odds ratio (OR) of 2. The T2D GRS meets this criteria for all common ancestry groups.
Clinical Impact and Prevalence
T2D affects hundreds of millions of adults worldwide,[2] with an estimated lifetime risk of 35.7% in the US.[9] This risk is strongly modified by obesity, diet, physical inactivity, and socioeconomic change.[2] Treatment of T2D focuses on lowering blood sugar and reducing long-term complications through a combination of lifestyle changes and medications. Weight loss, improved diet, and increased physical activity are foundational, while medications are often required to improve insulin action or reduce excess glucose. Multiple drugs are commonly used together as the disease progresses. Ongoing management also targets related risk factors such as blood pressure and cholesterol to reduce the risk of heart disease, stroke, and other complications.[2]
Performant Risk Stratification
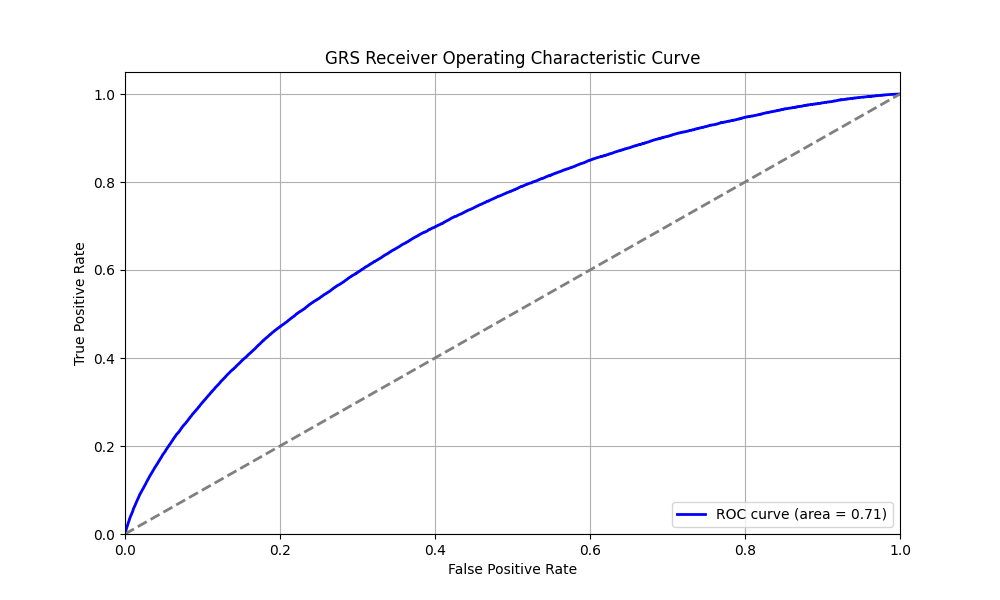
We evaluated the predictive performance of Orchid’s T2D GRS using the UK Biobank (UKB), a research database of roughly 500,000 genotyped individuals from the United Kingdom. We restricted the analysis to participants of British ancestry and defined T2D using the E11.x ICD-10 code, yielding 29,782 cases and 378,738 controls (7.3% prevalence). We then grouped individuals by GRS percentile and compared the observed disease prevalence within each group to our model’s predictions (Figure 1). For additional technical details, see the Supplementary Data.
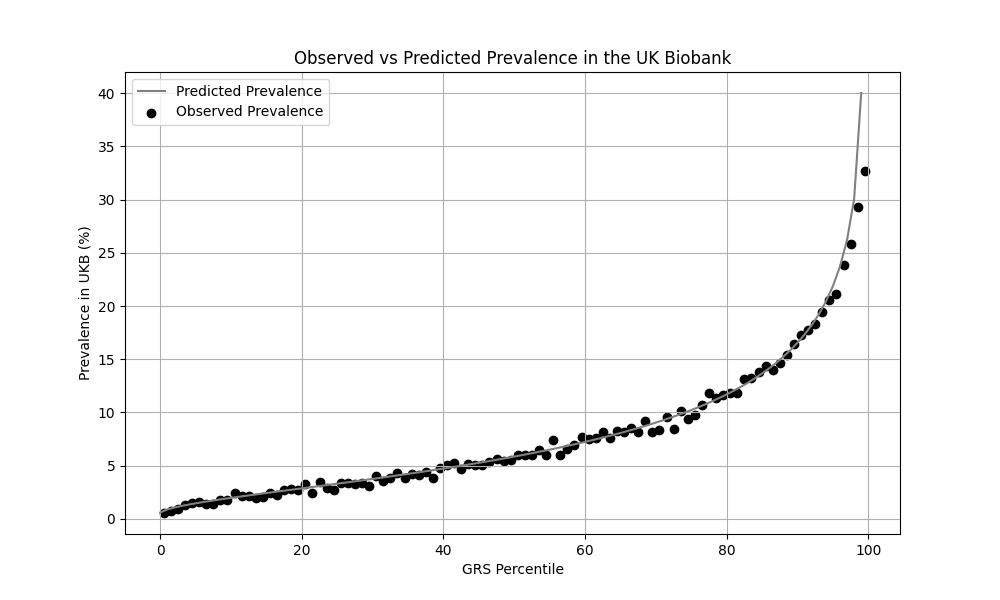
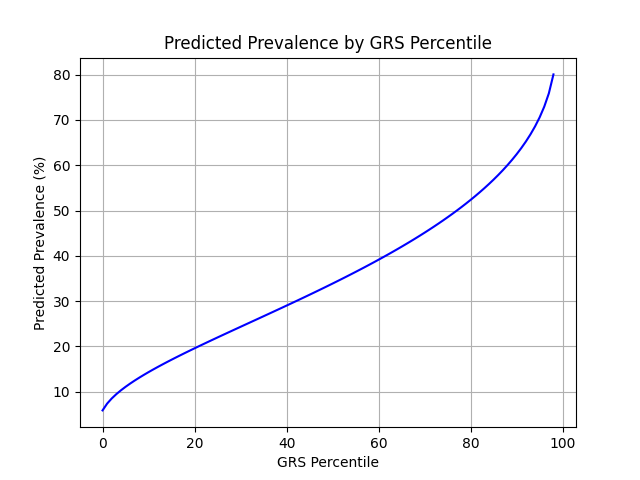
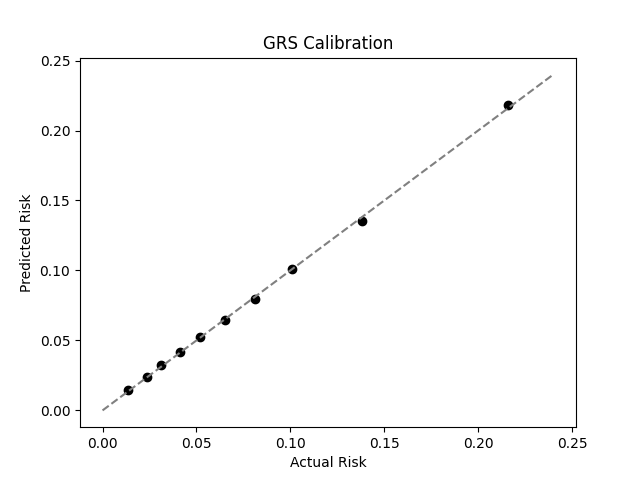
UKB participants tend to be healthier than the general population, which leads to lower observed disease prevalence.[10] Narayan et al. estimate a 35.7% lifetime risk of T2D, much higher than the prevalence in the UKB.[9] We adjust our model so that its average predicted risk aligns with this estimate (see Figure 2).[11] People at the tail end of the GRS distribution were at an elevated risk compared to the mean (see Table 3), with adults in the 99th percentile 2.2x more likely to develop T2D than average (80.1% vs 35.7%).
References
1. Centers for Disease Control and Prevention. Diabetes: Signs and Symptoms. Available at: https://www.cdc.gov/diabetes/signs-symptoms/index.html. Accessed 2025-12-05
2. DeFronzo R, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi:10.1038/nrdp.2015.19
3. Moore S, Davidson I, Anomaly J, et al. Development and validation of polygenic scores for within-family prediction of disease risks. medRxiv. 2025. doi:10.1101/2025.08.06.25333145.
4. Cordogan S, Starr DB, Treff NR, et al. Within- and between-family validation of nine polygenic risk scores developed in 1.5 million individuals: implications for IVF, embryo selection, and reduction in lifetime disease risk. medRxiv. 2025. doi:10.1101/2025.10.24.25338613.
5. Zheng, Z., Liu, S., Sidorenko, J. et al. Leveraging functional genomic annotations and genome coverage to improve polygenic prediction of complex traits within and between ancestries. Nat Genet 56, 767–777 (2024). https://doi.org/10.1038/s41588-024-01704-y
6. FinnGen. FinnGen+MVP+UKBB Summary Statistics. Available at: https://mvp-ukbb.finngen.fi/about. Accessed 2025-12-05.
7. FinnGen. FinnGen+MVP+UKBB Phenotypes. Available at: https://mvp-ukbb.finngen.fi. Accessed 2025-12-15.
8. Privé, Florian et al. “Portability of 245 polygenic scores when derived from the UK Biobank and applied to 9 ancestry groups from the same cohort.” American journal of human genetics vol. 109,1 (2022): 12-23. doi:10.1016/j.ajhg.2021.11.008
9. Narayan KMV, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi:10.1001/jama.290.14.1884.
10. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi:10.1093/aje/kwx246.
11. Chatterjee N, Shi J, García-Closas M et al. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17:392–406. doi:10.1038/nrg.2016.27
Supplementary Figures
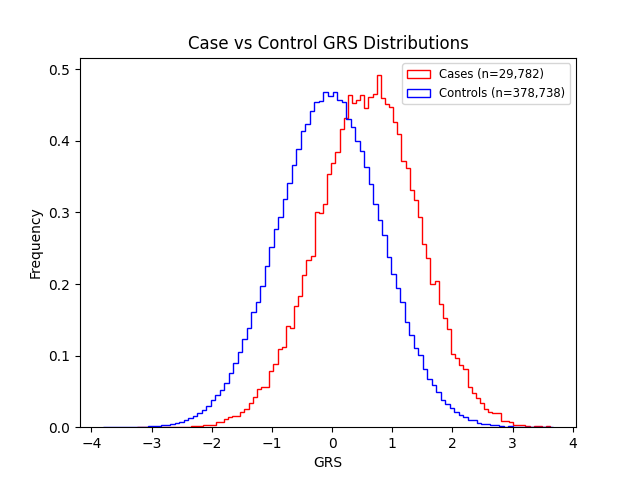
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 80545.